How is urea produced?
Commercially, urea, is produced by the direct dehydration of ammonium carbamate, NH2COONH4, at elevated temperature and pressure. Mr. Carl Bosch developed a chemical high pressure method during the establishment of the new ammonia industry and was awarded the Nobel Prize in 1932.
Urea is produced from NH3 and CO2 at high pressure and temperature; both reactants are obtained from an ammonia-synthesis plant. The latter is a by-product stream, vented from the CO2 removal section of the ammonia-synthesis plant. The two feed components are delivered to the high pressure urea synthesis section. Depending on the feed mol ratio, the water content and parameters such as pressure, temperature, and residence time, more or less carbamate is converted into urea and water.
Currently more than 500 urea plants are in operation worldwide producing together more than 200 million tons of urea each year. Over 90% of all new urea plants are licensed by Saipem, Stamicarbon, or Toyo Engineering Corporation. Saipem utilizes thermal stripping while Stamicarbon and Toyo Engineering Corporation use CO2 stripping.
What is important in urea production plants?
Nowadays plant capacities are more than 4000 metric tons per day in one line and plant sizes become bigger
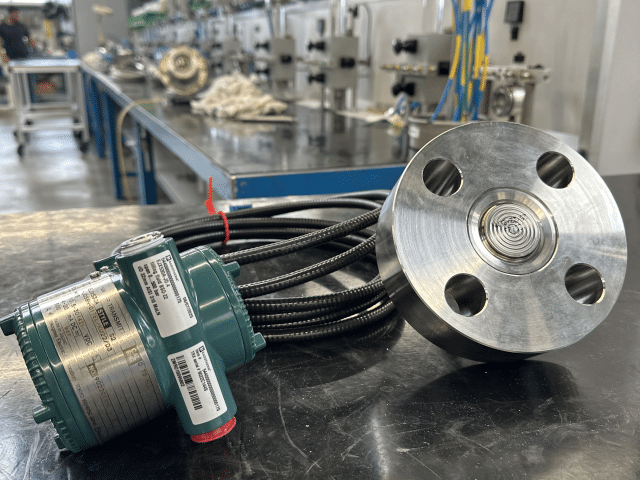
and bigger as a result from the economics of scale. A high reliability level and maximum on-stream figures are very important. BADOTHERM has been developing innovative solutions to contribute to the safety and reliability of urea plants by successfully solving the issues around pressure and level measurements in these urea plants.

Challenges with Materials
What are the corrosion challenges in urea plants?
Urea is made by the reaction of ammonia and carbon dioxide under a pressure of 150-220 bar, in a reactor at temperatures in the range 170-200°C. The corrosive conditions are severe in all high pressure parts where the intermediate component ammonium carbamate is present. Several factors govern the corrosion of stainless steel in carbamate solutions.
Corrosion rates are influenced by the temperature and the amount of carbamate, but of utmost importance is the oxygen content. If there is no oxygen present, stainless steels will corrode actively at high rates (picture on right side), whereas only small amounts of oxygen are sufficient to keep them in the passive state (picture on left side). The amount of oxygen necessary is determined by the type of steel being used. In operation, the oxygen addition must be much higher to ensure that there is a sufficient amount of oxygen in all parts of the plant.
Which materials have been applied in a urea plant?
Special stainless steel grades must be used in the high pressure urea process to achieve long service life. The process conditions are highly corrosive and as the corrosion mechanism is complex, the development of suitable alloys has been concentrated in the past on refining existing grades by continuous improvement. The use of these special “urea grades” has resulted in low maintenance costs and long service life. Proper control of the process, as well as high pressure equipment manufactured with high quality products, is also needed for a long service life.

Over the last 30 years, two austenitic stainless grades have been dominant in urea plants with stripper technology. The lower alloyed version is a modified ASTM 316L (UNS No 31603), referred to as 316L MOD or 316L Urea Grade. It is modified to meet the requirement of a maximum 0.6 % ferrite and a maximum corrosion rate in the Huey test of 0.60 mm/year. Both are needed to provide the low corrosion rate for this type of alloy. The commercial version of 316L does not meet these requirements, as the alloy elements as per codes are too low in combination with too high carbon.
For the more corrosive areas, a higher alloyed material is needed. It is referred to as 25.22.2, which stands for the chromium, nickel and molybdenum contents. With a chromium content raised from 17 to 22%, and increased nickel content from 14 to 22%, corrosion resistance was further improved compared with 316L Urea Grade. Also, 25.22.2 alloy has an international designation UNS S 31050 (AISI 310) WSN 1.4465 or WSN 1.4466
What is the best material?
Zirconium has proven to be most corrosion resistant under the most severe high pressure and temperature conditions in a urea plant. That is why BADOTHERM has developed innovative solutions for pressure and level measurements in the urea synthesis section of any urea plant based on zirconium and has established a world record on-stream time for a pressure measurement under the most critical thinkable condition.